How Does TB-500 Work?
Mechanism of Action Explained
Introduction:
How does TB-500 work? As a synthetic peptide derived from the core of thymosin β4, TB-500 accelerates tissue repair by modulating multiple healing pathways: cell migration, angiogenesis, stem cell recruitment, inflammation control, and cytoprotection¹
TB-500 Mechanism of Action: The Science Explained
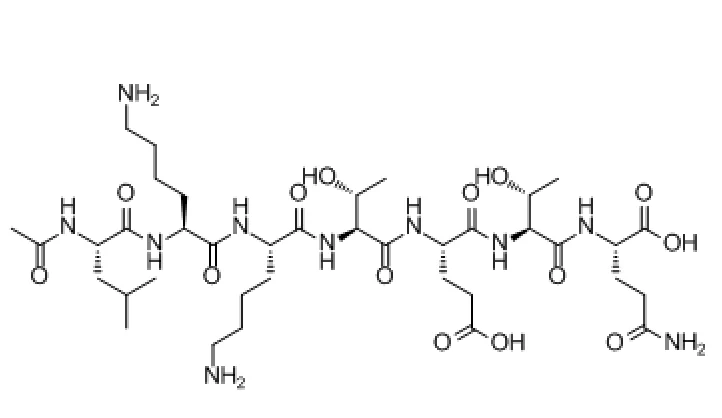
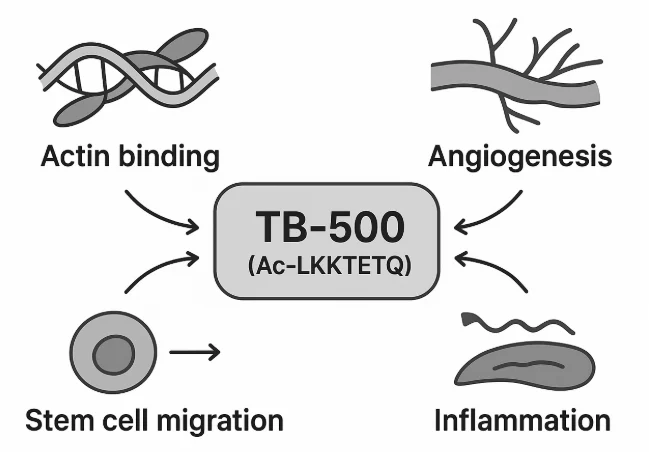
TB-500 does not act on a single receptor or pathway. Instead, it orchestrates a coordinated healing response by influencing several major biological systems involved in tissue regeneration¹.
The phrase “mechanism of action” refers to the biological processes through which a molecule produces its effects. In the case of TB-500, preclinical research demonstrates its ability to:
- Enhance cell migration by regulating actin dynamics¹
- Promote angiogenesis (formation of new blood vessels)²
- Recruit and differentiate stem/progenitor cells at injury sites³
- Modulate inflammation and minimize fibrotic (scar) tissue⁴
- Protect cells from programmed death and oxidative stress⁵
Importantly, no unique TB-500 receptor has been identified—it acts as a network modulator, coordinating multiple key processes required for effective repair.
1. Actin Binding & Cell Migration
TB-500 sequesters G-actin monomers, maintaining a readily available pool for actin filament assembly. This accelerates the migration of repair cells—fibroblasts, endothelial cells, and keratinocytes—into damaged tissue, facilitating rapid wound closure and tissue regeneration¹.
Why this matters: Faster, more efficient cell migration means shorter recovery times and improved tissue repair outcomes.
2. Angiogenesis & Tissue Healing
TB-500 upregulates VEGF (vascular endothelial growth factor) and stabilizes HIF-1α, driving the growth of new capillaries within injured tissues².
Why this matters: Enhanced blood supply supports robust recovery in muscle, tendon, skin, and even cardiac tissue.
3. Stem/Progenitor Cell Recruitment
TB-500 attracts stem and progenitor cells to sites of injury and supports their differentiation into specialized cell types, such as muscle fibers or blood vessel cells³.
Why this matters: A greater pool of regenerative cells at injury sites leads to more complete, tissue-specific healing.
4. Inflammation Modulation & Anti-Fibrosis
TB-500 reduces pro-inflammatory cytokines and suppresses myofibroblast activity, resulting in a faster transition from inflammation to repair and less scar tissue formation⁴.
Why this matters: Lower inflammation and minimal fibrosis yield stronger, more flexible healed tissue.
5. Cytoprotection & Anti-Apoptotic Activity
TB-500 upregulates anti-apoptotic proteins (e.g., Bcl-2) and antioxidant enzymes, helping cells survive oxidative stress and resist programmed death around injuries⁵.
Why this matters: Preserving more healthy cells near injury sites supports stronger and more complete regeneration.
TB-500’s Multi-Target Profile: Why It’s Unique
Unlike peptides that act on a single pathway, TB-500’s network effects—across actin regulation, angiogenesis, stem cell activity, inflammation control, and cytoprotection—make it uniquely versatile in regenerative research
Limitations: What Do Studies Say?
Most evidence for TB-500’s mechanisms comes from animal and cell studies. While findings are promising, direct human clinical research remains limited.
Conclusion
TB-500 works through a multi-target, multi-pathway approach: regulating actin for cell migration, driving angiogenesis, recruiting and differentiating stem cells, modulating inflammation, and protecting cells. This broad activity underpins its promise as a regenerative research peptide. Further human studies are needed to confirm its full clinical potential.
FAQs About TB-500 Mechanism
How does TB-500 promote healing?
By orchestrating cell migration, angiogenesis, stem cell activity, and inflammation control, TB-500 accelerates tissue repair at multiple biological levels.
Does TB-500 have a unique receptor?
No. TB-500 does not act via a unique receptor; its effects result from modulating established cellular pathways
Is TB-500’s mechanism proven in humans?
Most data comes from animal and cellular studies. Human clinical data are limited.
Can TB-500 be combined with other peptides?
TB-500 is often researched in combination with peptides like BPC-157 for synergistic healing effects, but this remains an area of ongoing research.
Related Articles
- What is TB-500
- TB-500 Side Effects & Safety
- TB-500 Benefits
- BPC-157 vs TB-500 vs GHK-Cu
- How Does BPC-157 Work
- How Does GHK-Cu Work
References
- Goldschmidt-Clermont PJ, Furman MI, Wachsstock D, Safer D, Nachmias VT, Pollard TD. The control of actin nucleotide exchange by thymosin β4 and profilin: a potential regulatory mechanism for actin polymerization in cells. Mol Biol Cell. 1992;3(9):1015–1024. “Thymosin β4 regulates actin polymerization by controlling nucleotide exchange on actin monomers.” https://pmc.ncbi.nlm.nih.gov/articles/PMC275662/
- Jo JO, Kim SR, Bae MK, Kang YJ, Ock MS, Kleinman HK, Cha HJ. Thymosin β4 induces the expression of vascular endothelial growth factor (VEGF) in a hypoxia-inducible factor (HIF)-1α-dependent manner. Biochim Biophys Acta. 2010 Nov;1803(11):1244-51. doi: 10.1016/j.bbamcr.2010.07.005. Epub 2010 Aug 4. PMID: 20691219. https://pubmed.ncbi.nlm.nih.gov/20691219/
- Zhao Y, Song J, Bi X, Gao J, Shen Z, Zhu J, Fu G. Thymosin β4 promotes endothelial progenitor cell angiogenesis via a vascular endothelial growth factor-dependent mechanism. Mol Med Rep. 2018;17(6):2314–2320. “Tβ4 enhances angiogenesis through VEGF signaling in endothelial progenitor cells.” https://www.spandidos-publications.com/10.3892/mmr.2018.9199
- Xing Y, Ye Y, Zuo H, Li Y. Progress on the function and application of thymosin β4. Front Endocrinol. 2021;12:767785. “Comprehensive review of thymosin β4’s roles in tissue repair, angiogenesis, and clinical potential.” https://www.frontiersin.org/articles/10.3389/fendo.2021.767785/full
- Kumar S, Gupta S. Thymosin β4 and protection against oxidative stress: upregulation of Bcl-2 in cardiomyocytes. PLoS ONE. 2011;6(8):e26912. “Thymosin Beta 4 Prevents Oxidative Stress by Targeting Antioxidant and Anti-Apoptotic Genes in Cardiac Fibroblasts” https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0026912