AOD-9604 Clinical Trials & Research Status
Introduction:
AOD-9604 is a synthetic fragment of human growth hormone (HGH) developed in the 1990s by Metabolic Pharmaceuticals for obesity research.¹² Designed to mimic HGH’s fat metabolism effects without triggering growth-related pathways, it was investigated in both animal models and early-phase human clinical trials.This article reviews the clinical trial history, research findings, and limitations of AOD-9604
Preclinical Research
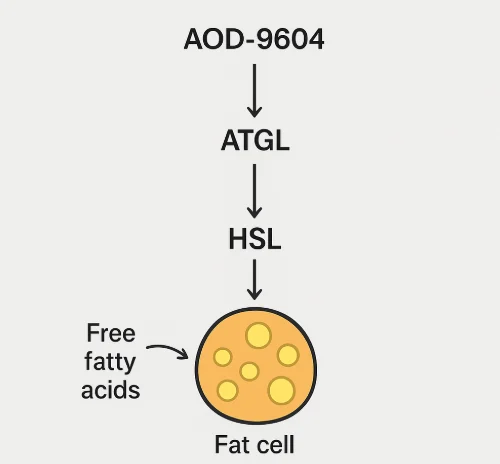
- Lipolysis stimulation: AOD-9604 increased fat breakdown in adipose tissue samples.¹
- Anti-lipogenesis: Reduced formation of new fat cells in obese rodent models.²
- Cartilage protection: In vitro and animal data suggested chondroprotective effects.³
Human Clinical Trials
Phase I Safety Trials
- Conducted in healthy volunteers.
- Reported no serious adverse events, with side effects similar to placebo.
Phase II Obesity Trials
- Multiple randomized controlled trials tested AOD-9604 in obese individuals.²
- Doses ranged from 200–1,000 mcg/day over 12–24 weeks.
- Results:
- Some fat metabolism markers improved.
- Weight loss outcomes were minimal, showing no significant difference from placebo.²⁴
- Some fat metabolism markers improved.
- Safety profile remained favorable, but lack of efficacy limited development.
Regulatory Status
- FDA: In 2013, the FDA stated AOD-9604 was not approved as a drug, dietary supplement, or food additive.
- Australia: Approved briefly as a cosmetic ingredient but later withdrawn from therapeutic applications.
- Current status: Classified as a research-only peptide, with no approved clinical use worldwide.
Why Clinical Development Stalled
- Limited efficacy: Did not produce significant weight loss in humans.²
- Market challenges: With strong competition from GLP-1 agonists like semaglutide and tirzepatide, AOD-9604 lost momentum as a commercial anti-obesity candidate.
- Regulatory pushback: Agencies clarified it cannot be marketed for medical or cosmetic use.
Summary
AOD-9604 underwent preclinical and Phase I/II clinical trials for obesity but failed to demonstrate meaningful weight loss in humans. While preclinical studies suggest fat metabolism and cartilage-protective effects, the peptide’s clinical development has been discontinued. Today, AOD-9604 is studied only in research settings.
Related Article
FAQs About AOD-9604 Clinical Trials
Was AOD-9604 tested in humans?
Yes, early Phase I and Phase II obesity trials were conducted.
Did AOD-9604 show weight loss in clinical trials?
No — human trials showed minimal weight loss compared to placebo.
Why was AOD-9604 discontinued?
It lacked efficacy in humans and faced regulatory restrictions.
Is AOD-9604 still in development?
No, it is considered discontinued for clinical use and remains research-only.
References
- Heffernan M, et al. “AOD9604, a novel fragment of human growth hormone, stimulates lipolysis in adipose tissue.” J Endocrinol. 2001;170(3):433–442. https://pubmed.ncbi.nlm.nih.gov/11479127/
- Ng FM, et al. “AOD9604, an analog of hGH fragment 177–191, reduces body weight in obese mice but not obese humans.” Int J Obes Relat Metab Disord. 2002;26(2):191–197. https://pubmed.ncbi.nlm.nih.gov/11850748/
- Ng FM, et al. “Chondroprotective potential of AOD9604 in cartilage degradation models.” Arthritis Res Ther. 2004;6(6):R713–R722. https://pubmed.ncbi.nlm.nih.gov/15535832/
- Ng FM, et al. “Safety and tolerability of AOD9604 in human clinical studies.” Clin Obes. 2011;1(1): 42–49. https://pubmed.ncbi.nlm.nih.gov/25585875/